Marksans Pharma shares jump 11% on USFDA nod for allergic drug
The company said that USFDA has granted not for an Abbreviated New Drug Application (ANDA) for Loratadine liquid filled capsules 10 mg.

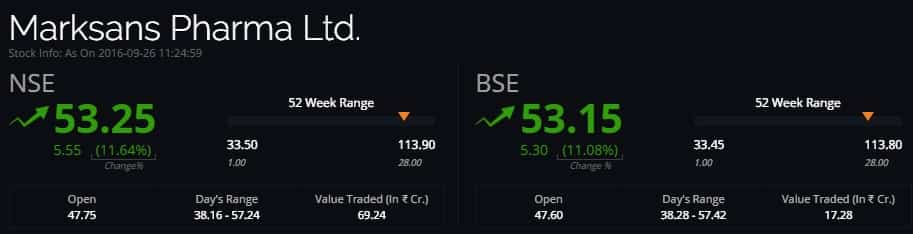
The shares of Marksans Pharma on Monday surged nearly 11% after the company got approval from US Food and Drug Administration (USFDA) for an allergic drug.
At 1145 hours the shares of the company were trading at Rs 53.10 per piece, or 10.97%, Rs 5.25 on BSE. The shares hit a high at Rs 53.85 and low at Rs 47.45.
In a regulatory filing, the company said that USFDA has granted not for an Abbreviated New Drug Application (ANDA) for Loratadine liquid filled capsules 10 mg.
"Loratadine Liquid Filled Capsules 10mg is therapeutically equivalent to the reference listed drug Claritin Liqui-Gels capsules 10mg of Bayer Healthcare LLC."
Loratafine is an antihistamine that reduces the effects of natural chemical histamine in the body. It is used to treat sneezing, runny nose, watery eyes, hives, skin rash, itching and other allergy symptoms, the company said in the filing.
Get Latest Business News, Stock Market Updates and Videos; Check your tax outgo through Income Tax Calculator and save money through our Personal Finance coverage. Check Business Breaking News Live on Zee Business Twitter and Facebook. Subscribe on YouTube.
11:58 AM IST










 Marksans Pharma soars over 14.65% after MIT buys its 66 lakh shares; multibagger stock has risen over 126% in 1 year
Marksans Pharma soars over 14.65% after MIT buys its 66 lakh shares; multibagger stock has risen over 126% in 1 year  Marksans Pharma to double its manufacturing capacity – here’s why? Check analyst view, management’s commentary, share price history and more
Marksans Pharma to double its manufacturing capacity – here’s why? Check analyst view, management’s commentary, share price history and more Mukul Agrawal stock: This small-cap pharma company share price zooms 8% – Here’s why
Mukul Agrawal stock: This small-cap pharma company share price zooms 8% – Here’s why  Marksans Pharma Share Price: What's moving this pharma stocks - know all TRIGGERS here
Marksans Pharma Share Price: What's moving this pharma stocks - know all TRIGGERS here